Research Interests
A: Molecular Pathogenesis of Hepatocellular Carcinoma and Nanovesicle Mediated Targeted Drug Delivery
Hepatocellular carcinoma (HCC) is a primary malignancy of the liver and is highly resistant to chemotherapy. The pathogenesis of HCC involves several different potential etiologies and can be associated with diverse genetic alterations. Currently, there is no effective treatment for advanced HCC and the available treatment methods are inadequate due to extensive genomic aberrations and heterogeneity of malignant hepatic tumors. The major problem facing cancer therapy is effective delivery of anticancer agents to the target organ. Development of nanoparticle mediated targeted drug delivery could solve this problem to an extent. Targeted drug delivery could be used for intracellular delivery of anticancer agents to a specific tissue or organ of interest, controlled release of drugs, enhanced systemic delivery, and optimized bioavailability. In addition, encapsulation of therapeutic agents would protect against metabolic and enzymatic degradation of drugs. If the nanoparticles are of biological origin and also biodegradable after their assigned function, it would be ideal for systemic delivery without eliciting host immune response.
Extracellular vesicles or exosomes are nanovesicles of 80–200 nm size that are released from most cell types in the body. Exosomes are present in most biological fluids and can be isolated using series of ultracentrifugation. There is considerable evidence that cell derived nanovesicles serve as vehicle for intercellular communication and transfer of cytosolic proteins, lipids, mRNAs, and miRNAs across cells and subsequently mediate changes in gene expression in the recipient cells. Since nanovesicles can stably carry cargo including chemotherapeutic agents and can pass through stringent biological barriers including blood brain barrier without eliciting immune response, they are considered as an ideal cellular vehicle for targeted drug delivery.
We have isolated nanovesicles from bovine skim milk (MNV), purified, and characterized using nanoparticle tracking analysis (NTA). MNVs are non-immune, non-toxic, and biocompatible with a suitable particle size ranging from 100-200 nm. Due to its appropriate and uniform size, MNVs can easily to enter into circulation and pass through cell membranes. We have demonstrated that chemotherapeutic agents and antisense oligonucleotides for miRNAs implicated with HCC could be successfully loaded into MNVs and can be selectively delivered into intrahepatic tumors through intravenous system. Since tumor cells evade immunosurveillance and their doors are widely opened to circulation compared to normal cells, the targeted delivery of MNVs and their cargo into the tumor cells could be possible with minimal affects to normal cells. The presence of leaky blood vessels and the lack of proper lymphatic drainage in solid tumors could favor extravasation and retention of exosomes inside the tumor cells. Therefore, the successful development of nanovesicle mediated targeted delivery of anticancer agents could be an efficient modality for the treatment of malignant HCC and might produce a great impact on anticancer therapy.
(Laboratory Investigation 2018; 98: 895-910 PDF, Seminars in Liver Disease 2015; 35: 63-74 PDF, Digestive Diseases and Sciences 2013; 58: 1923-1933 PDF).

B: Molecular Pathogenesis of Hepatic Fibrosis and Prospective Therapeutic Approaches
Hepatic fibrosis and alcoholic cirrhosis are chronic diseases and serious health problems worldwide. Hepatic fibrosis is characterized by excessive synthesis and deposition of connective tissue proteins, especially interstitial collagens in the extracellular matrix of the liver. It is a dynamic process results in continuous wound healing response to a variety of chronic stimuli, such as ethanol, viruses, toxins, drugs, or cholestasis. The chronic stimuli that involved in the initiation of fibrosis leads to oxidative stress and generation of reactive oxygen species (ROS) that serve as mediators of molecular events involved in the pathogenesis of hepatic fibrosis. These processes lead to cellular injury and trigger inflammatory responses releasing a variety of cytokines and growth factors that initiate activation and transformation of resting hepatic stellate cells into myofibroblast like cells, which in turn start excessive synthesis of connective tissue proteins, especially collagens. Uncontrolled and extensive fibrosis results in distortion of lobular architecture of the liver leading to nodular formation and cirrhosis. The repeated injury and regeneration process could also results in genomic aberrations and mutations that lead to the development of hepatocellular carcinoma (HCC). Regulation of the several steps involved in the activation and transformation of hepatic stellate cells offers a potential therapeutic target for the arrest of hepatic fibrosis and liver cirrhosis.
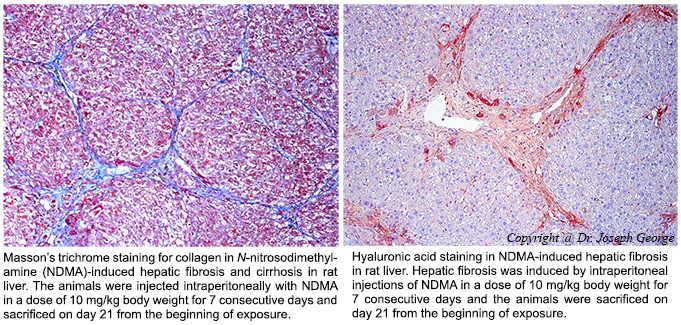
Connective tissue growth factor (CTGF) is a multifunctional protein that regulates a diverse array of cellular functions including wound healing, tissue remodeling, and fibrosis. CTGF is a potent, highly profibrogenic molecule that plays a significant role in the pathogenesis of hepatic fibrosis. CTGF expression is very low in normal liver but highly upregulated in fibrotic liver and produced by multiple cell types in the liver, mainly the activated hepatic stellate cells. It is one of the molecules that trigger the activation and transformation of quiescent hepatic stellate cells into myofibroblasts. It also stimulates production of more CTGF by autocrine mechanism. Overall, CTGF is one of the potent molecules that trigger excessive production of extracellular matrix proteins and promotes hepatic fibrogenesis.
Transforming growth factor-β1 (TGF-β1) is a multifunctional cytokine involved in various pathophysiological processes including regulation of cell growth, cell proliferation, cell differentiation, apoptosis, angiogenesis, and fibrosis. TGF-β1 is the most prominent molecule that triggers the synthesis of connective tissue components during pathogenesis of hepatic fibrosis. It also stimulates the production of protease inhibitors such as tissue inhibitor of metalloproteases (TIMPs) that prevent degradation of extracellular matrix proteins. TGF-β1 induces the production of CTGF, which in turn triggers the production of more TGF-β1. The related expression patterns of CTGF and TGF-β1 indicate that the two molecules act synergistically in the synthesis and deposition of extracellular matrix proteins in the hepatic parenchyma leading to fibrosis and cirrhosis. Strategies that block the upregulation of both CTGF and TGF-β1 offer promising modalities to prevent activation of hepatic stellate cells and thus hepatic fibrosis, liver cirrhosis, and HCC.
(Journal of Molecular Medicine 2020; 98: 1203-1213. PDF, Scientific Reports 2019; 9: 708 PDF, Cell Death & Disease 2019; 10: 18 PDF, Biological Chemistry 2018; 399: 499-509 PDF, Journal of Cellular and Molecular Medicine 2017; 21: 3821-3835 PDF, Gene Therapy 2007; 14: 790-803 PDF).

C: Tissue Engineering and Regenerative Medicine Involving Stem Cells
The use of tissue engineered biological substitutes employing living cells is emerging as an alternative to conventional tissue or organ transplantation. Using this technology, tissue loss or organ failure can be treated by implantation of an engineered biological substitute that is either functional at the time of implantation or has the potential to integrate and form the expected functional tissue or organ at a later stage. Three dimensional (3-D) cell cultures on biodegradable scaffolds are the basis of tissue engineering, where the specific cells can grow and multiply into a structure similar to tissues or organs in the living body. Collagen-hyaluronan-based biodegradable scaffolds are an excellent substratum for cell adhesion, differentiation and proliferation. The application of biodegradable 3-D scaffolds in the field of tissue engineering and regenerative medicine is highly promising.

Potential Applications
Adult mesenchymal and cord blood stem cells are pluripotent, with the ability to differentiate into multi-lineage cells such as neurons, adipocytes, chondrocytes or osteoblasts when cultured in a special media with appropriate growth factors. The differentiated cells on a 3-D scaffold have the potential to form a variety of mesenchymal tissues such as cartilage, tendon, ligament, muscle or adipose tissue. Surgical implantation of such artificial organs derived from human stem cells has the potential to replace the impaired or damaged tissue or organ. The 3-D culture of hepatocytes on a specially designed collagen scaffold offers a method to develop a functional artificial liver. Identification and isolation of pluripotent stem cells and subsequent injection into the human body could regenerate the impaired or damaged tissue or organ. Isolation of embryonic or adult human stem cells for β-cells of pancreas and subsequent injection into the site could rejuvenate islets of Langerhans and start insulin production in diabetic patients (Journal of Biomedical Materials Research 2008; 87A: 1103-1111 PDF, Biotechnology and Bioengineering 2006; 95: 404-411 PDF). |